Coronavirus update: Trump quits WHO in multi-front battle vs. China; NYC eyes June 8 reopen
In the midst of rising tensions with China and the World Health Organization, President Donald Trump on Friday announced that the U.S. was revoking its support for the agency, the latest escalation in a battle that has seen the president accuse the WHO of being beholden to Beijing, and failing in its obligations to contain the coronavirus pandemic.
The WHO has come under heavy fire from the U.S. for being slow to declare COVID-19 a global crisis, and being perceived as too deferential to China, where the outbreak originated.
“Chinese officials ignored their reporting obligations to the World Health Organization, and pressured the World Health Organization to mislead the world when the virus was first discovered by Chinese officials,” Trump told a news conference, where he also announced measures designed to punish Beijing over its provocations against Hong Kong.
“China has total control over the WHO, despite only paying $40 million per year, compared to what the U.S. has been paying, which is approximately $450 million a year,” Trump said.
“Because they have failed to make the requested and greatly needed reforms, we will be today terminating our relationship with the World Health Organization and redirecting those funds to other worldwide and deserving urgent global public health needs,” the president added.
“The world needs answers from China on the virus. We must have transparency,” he said.
But the bold move by Trump upset a number of top health experts in the country.
Dr. Atul Gawande, who heads Haven, the health venture between Amazon, Berkshire Hathaway and J.P. Morgan, tweeted the move was a “disaster.”
“Pulling out of WHO is a disaster for the lives and health of all people, including Americans. I can’t imaging a worse thing to do in the midst of a pandemic and ongoing work to fight back TB, HIV, polio and other health threats. America First does not work for global disease,” Gawande said.
Similarly, Dr. Tom Tsai, faculty and collaborator with the Harvard Global Health Institute, noted that details about the termination of the relationship were still unknown.
“President Trump has a history of making announcements without any clear policy guidance of what he means,” Tsai said.
But what is clear, he said, is that “the U.S. is abandoning a global effort to combat the pandemic at a time where it’s more crucially needed. The rest of world is still marching on, in terms of collaboration and cooperation. That mean the U.S. may be left behind to face this on its own— and we’ve clearly seen the failure of that approach.”
Separately, New York City was poised to mark a major turning point in the global crisis, with the city targeting June 8 to join other regions in relaxing the restrictions that have throttled the world’s largest economy.
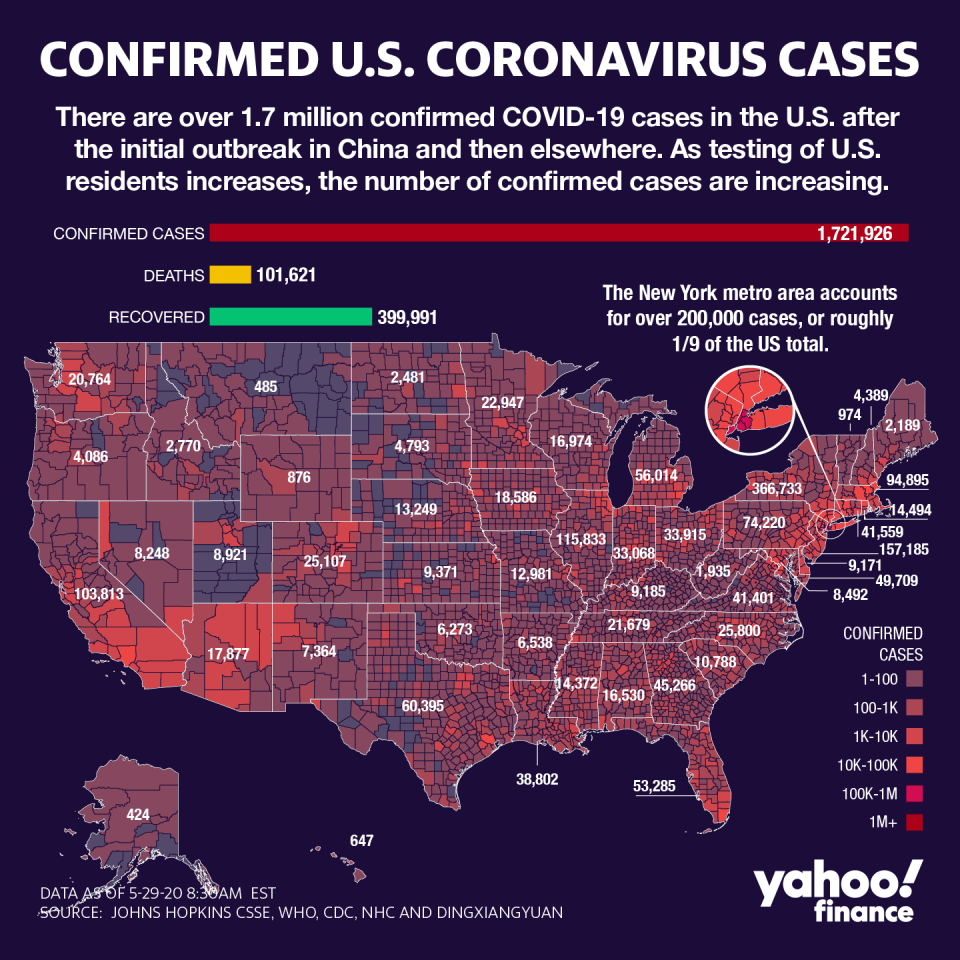
Up until very recently, the Big Apple was considered one of the world’s largest epicenters of the COVID-19 pandemic, which has infected nearly 6 million worldwide and killed over 362,000. In the U.S., more than 1.7 million cases have been reported with more than 101,000 dead, but the Empire State’s daily death count hit a new low of 67 on Thursday.
Yet with hospitalizations and new infections on a down-curve, New York Gov. Andrew Cuomo said the city would begin unwinding stay-at-home orders — among the nation’s strictest — with NYC-based businesses eyeing early June for a return to work. Meanwhile, CNBC reported that Wall Street banking giant Morgan Stanley will allow traders to come back to the office next month.
The developments come as the Centers for Disease Control unveiled wide-ranging office guidelines to prevent new infections, but are seen as having a dramatic impact on workplace culture. Both the CDC and the Empire State’s governor urged citizens to continue wearing masks, and abiding by social distancing measures to prevent further spreading.
“Those simple devices...make all the difference,” Cuomo told reporters on Friday. “Getting 19 million people to do it, that’s what’s hard.”
More debate over COVID drug treatment
As more pharmaceutical companies rise to the challenge of finding effective COVID-19 treatments and a potential vaccine, new controversy was stirred over hydroxychloroquine, an anti-malarial generic that’s been used in coronavirus trial treatments.
Reuters reported on Friday that Sanofi (SNY) has temporarily stopped recruiting new COVID-19 patients for two clinical trials on hydroxychloroquine, and will no longer supply the drug until concerns about safety are cleared up.
The drug has been the center of a media firestorm, especially after President Donald Trump announced he was using it as a preventative measure. Last week, an article in The Lancet article, a medical journal, prompted the World Health Organization to halt its trials on hydroxychloroquine.
However, that same article — which said the drug was ineffective and deadly — has been questioned by experts, with more than 100 doctors from around the world signing a letter that pushed back on its conclusions.
“The subsequent media headlines have caused considerable concern to participants and patients enrolled in randomized controlled trials...This impact has led many researchers around the world to scrutinize in detail the publication in question. This scrutiny has raised both methodological and data integrity concerns,” the letter said.
It isn’t the first time experts have doubted The Lancet’s conclusions. It also published the results of clinical trials of Gilead Science’s antiviral treatment in China, which were halted due to a lack of sufficient participants. The article stated the treatment, remdesivir, was ineffective.
However, data from the National Institute of Health showed the treatment had a positive effect. But even those results had critics, saying the improvement seen in treated patients was insignificant and detailed data was missing.

 money
money 
